To manufacture, market, import or spend cosmetic products in Colombia, as a member country of the Andean Community, a mandatory health notification is required, which is an alphanumeric code issued by INVIMA. It is pertinent to clarify that the
obligatory sanitary notification
is equivalent to sanitary registration, according to the final provision of Decision 516 of 2002. This regulation is part of the block of harmonization of legislation on Cosmetic Products of the Commission of the Andean Community of National of which Colombia is part and whose regulations are fully applicable in its territory.
The regulation of the mandatory sanitary notification is found in Decision 516 of 2002, which was amended and complemented by Decision 833 of 2018.
What is meant by cosmetics?
Article 1 of Decision 516 of 2002 defines them as:
Any substance or formulation of local application to be used on the various superficial parts of the human body: epidermis, hair and capillary system, nails, lips and external genital organs or on the teeth and oral mucous membranes, in order to clean, perfume, modify their appearance and protect or maintain them in good condition and prevent or correct body odors.
How are cosmetics classified?
Annex 1 of Decision 516 of 2002 establishes an indicative list of categories of products that are considered cosmetics, such as:
a) Children’s cosmetics. b) Cosmetics for the eye area. c) Skin cosmetics. d) Lip cosmetics. e) Cosmetics for body grooming and hygiene. f) Deodorants and antiperspirants. g) Hair cosmetics. h) Nail cosmetics. i) Perfumery cosmetics. j) Oral and dental hygiene products. k) Products for and after shaving. l) Tanning products, sunscreen and self-tanning products. ll) Depilatories. m) Skin bleaching products.
Additionally, CAN Resolution 1906 of 2017 incorporates the following harmonized list of cosmetic forms:
- Oil: Substance immiscible with water, greasy, viscous and liquid at room temperature.
- Aerosol: Pressurized form containing ingredients that are released under activation of the valve system to disperse liquids, solids or gases.
- Bar: solid mixture that is applied by sliding.
- Compact solid: solid mixture that needs to be dissolved for its application.
- Wax: mixture of fatty nature; natural or synthetic; solid or semi-solid.
- Emulsion: heterogeneous system of two or more phrases, of semisolid or fluid consistency, stabilized with an emulsifier.
- Cream gel: colloidal system where the continuous phase is solid and the dispersed phase is liquid to which small amounts of fat are added and are at the limit of emulsions.
- Enamel: solvent-based mixture which, when applied to a dry surface, forms a covering film.
- Gel: colloidal system, where the continuous phase is solid and the dispersed phase is liquid.
- Granules: small particles that are usually dissolved or dispersed in aqueous or heterogeneous media.
- Pencil: solid paste compacted into a cylinder that imitates the shape of a common pencil, which is applied to the skin.
- Lotion: mixture or combination of two or more phases of fluid consistency with water as the main component, which may also contain alcohol and glycols, containing the dispersed or diluted solute.
- Impregnated Support: base that acts as a support on which the cosmetic mixture or substance is impregnated.
- Paste: semi-solid form with a high content of finally dispersed solids, with a firm consistency.
- Pearls: soft gelatin or polymer containers containing oily or aqueous solutions.
- Powder: a mixture of loose or compacted solids, finally divided. A mixture of finally divided, loose or compacted solid particles.
- Ointment: substance of a greasy nature for application to the skin. The fundamental difference with emulsions is the absence of water.
- Solution: mixture of one or more solutes in one or more solvents.
- Suspension: dispersed system consisting of an insoluble solid phase in a liquid or semi-liquid phase.
Which products require mandatory health notification?
Article 5 of Decision 516 of 2002 affirms the mandatory nature of the sanitary notification for the commercialization of a cosmetic product, as follows:
The cosmetic products referred to in this Decision require, in order to be marketed or sold in the Subregion, the Mandatory Health Notification submitted to the Competent National Authority of the first Member Country of commercialization. Products manufactured in the Subregion must undergo the Mandatory Health Notification in the Member Country of manufacture prior to their commercialization.
What are the requirements to apply for a health notification?
On the official INVIMA website in the section on procedures and requirements to carry out a sanitary notification, some general, legal and technical requirements that must be met in order to proceed with the notification are indicated. However, the procedure can be done in person at the INVIMA offices or, virtually, online.
virtually
.
General Requirements:
- Consignment (Consignment slips should be pasted completely on a sheet of paper with glue or bound as a sheet. Do not staple)
- Basic information forms, application or, if applicable, letter of request.
- Legal information.
- Technical information.
Legal requirements for domestic products:
The manufacture of cosmetic products in the national territory must be carried out in an establishment authorized with production capacity by INVIMA, through a visit to the establishment’s facilities where the sanitary regulatory requirements are verified and a sanitary concept is issued in order for the establishment to start manufacturing activities. The following documents are also required:
- Completely filled out and signed form. (Must be signed by a pharmaceutical chemist with professional card, based in Colombia).
- Payment of the established fee.
- Product name and brand name if applicable. (It may cover several trademarks for the same Sanitary Notification, but keeping the name of the product).
- Cosmetic group, if applicable. (Read article 10 of Decision 516 of 2002) In this case you must take into account that you can group, in the case of cosmetic creams, only products with different scents or colors, but identical product name, uses and benefits.
- Cosmetic form. (gel, liquid, cream, emulsion, liquid, etc.).
- Manufacturing contract with the manufacturer, or conditioner; if applicable.


Legal requirements for imported products:
- Completely filled out and signed form. (Must be signed by a pharmaceutical chemist with a professional card and residing in Colombia).
- Payment of the established fee.
- Product name and brand name if applicable. (It may cover several trademarks for the same Sanitary Notification, but keeping the name of the product).
- Cosmetic group, if applicable. (Read Article 10 of Decision 516 of 2002) In this case you must take into account that you can group, in the case of cosmetic creams, only products with different scents or colors, but identical product name, uses and benefits.
- Cosmetic form. (Gel, liquid, cream, emulsion, liquid, etc.).
- Manufacturing contract with the manufacturer, or conditioner; if applicable.
- Apostilled certificate of free sale or similar authorization issued by the competent authority of the country of origin, this must not be older than five years from the date of presentation of the NSO.
- Authorization from the manufacturer to the person responsible for commercialization, indicating whether he/she authorizes him/her only to import the product or to be the holder of the Sanitary Notification.
Technical requirements for national products:
- Basic and secondary qualitative formula in INCI nomenclature. (Name of the ingredient in INCI nomenclature and the percentage of the ingredient in the formulation). The INCI nomenclature is an international nomenclature for ingredients.
- Quantitative formula for substances of restricted use and active ingredients with parameters established in INCI nomenclature. (You should consult the ingredients in the international listings. The ingredient lists of the Food & Drug Administration of the United States of America, The Personal Care Products Council and the European Union Directives are recognized for this purpose).
- Organoleptic and physicochemical specifications of the finished product. (These are determined by the manufacturer)
- Microbiological specifications. These must be submitted under the parameters of Resolution 1482 of July 2012.
- Instructions for use of the product.
- Precautions for use of the product, when applicable.
- Justification of goodness and cosmetic claims attributable to the product, whose untruthfulness may represent health problems and justification of goodness. Please note that the cosmetic product cannot be granted therapeutic properties.
- Draft label artwork (comply with the provisions of Article 18-22 of Decision 516 of 2002).
- Primary packaging material(what the packaging is made of in contact with the product).
What does the sanitary registration process consist of?
Article 10 of Decision 833 of 2018 establishes the process for the processing of the mandatory health notification. It is important to note that no lawyer is required.
After the requested documents are filed, INVIMA will review that all the requirements demanded by Article 9° of Decision 833 of 2018 are met; if so, an identification code will be assigned for labeling and market surveillance and sanitary control purposes automatically. On the contrary, if the authority evidences that the notification is not accompanied by the required documents, the code will not be granted.
What are the costs of the mandatory notification process?
The
Resolution 2019058384 dated December 27, 2019
updates the fees managed by INVIMA for all types of procedures, including the mandatory sanitary notification in terms of
Tax Value Unit (UVT)
where each unit has a value of 35,607 pesos for the year 2020. The following is the table of rates contained in the aforementioned resolution:
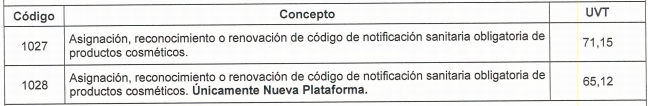

1027: 2, 533,438
1028: 2, 318,727
What is the validity of the mandatory health notification?
Article 18° of Decision 833 of 2018 states that the term of validity of the NSO shall be seven years, counted from the date of assignment of the NSO’s identification code.
How is the renewal of the mandatory health notification processed?
Article 18° of Decision 833 of 2018 establishes that the NSO may be renewed for the same period, successively with the code initially assigned. It also states the following procedure for renewal:
The NSO holder, before the expiration of the term of validity, shall submit its renewal request, attaching a sworn statement indicating that the product will continue to be marketed with the current requirements of Article 9 of this Decision, in addition to the proof of payment of the fee established by each Member Country. If such renewal is not carried out, the NSO identification code will be considered expired and cannot be assigned again.
Our lawyers are experts in the processing of the mandatory notification before the INVIMA of cosmetics and can provide you with the help and advice you require, if you want to know more about our services you can contact us through our website.



